Detail introduction: Icatibant/HOE 140/Icatibatide Acetate/130308-48-4
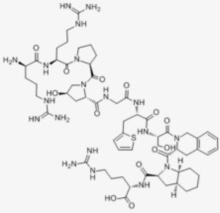
| English name | Icatibant |
| CAS NO | 130308-48-4 |
| Peptide sequence | DArg-Arg-pro-Hyp-Gly-Thi-Ser-DTic-Oic-Arg |
| Molecular formula | C59H89N19O13S |
| Molecular weight | 1304.5 |
| storage temperature | 2-8℃ |
| purity | ≥98% |
| Package | 1mg;5mg;10mg;50mg;100mg,1g or according to customer’s detail requirement. |
| Product English synonyms | HOE 140;Icatibatide Acetate;Icatibant;D-Arg-[Hyp3,Thi5,D-Tic7,Oic8]bradykinin |
Product introduction
Icatibant is a chemical with the molecular formula C59H89N19O13S. Icatibant is a HEA specific drug developed by the Shire. On November 7, 2008, this product was first approved as an orphan drug by EMEA under the trade name Firazyr for the treatment of acute attacks of adult hereditary angioedema (HAE).
It was approved by the U.S. FDA on August 25, 2011. It is the third drug approved by the FDA for the treatment of acute HAE attacks.
Icatibant is a selective, competitive antagonist for bradykinin B2 receptors, and its affinity is similar to bradykinin. Hereditary angioedema is caused by the lack or dysfunction of C1-esterase-inhibitors. Bradykinin is a vasodilator, which is believed to be responsible for the localized swelling, inflammation, and pain characteristic symptoms of HAE.
Icatibant treats the clinical symptoms of HAE acute attack by inhibiting the binding of bradykinin and B2 receptor. In addition, he has potential therapeutic indications such as asthma, liver cirrhosis and other types of angioedema.
Pharmacokinetic properties of HOE 140
30 minutes after subcutaneous injection of 30 mg of this product, the peak blood concentration can be reached, and the absolute bioavailability is close to 97%. After 30 minutes of injection of this product (0.4mg·kg-1) in young healthy men, the average volume of distribution is 0.25L·kg-1, the plasma protein binding rate is less than 44%, the average t1/2 is 0.6~1.5h, and the terminal half-life is 1.2~1.5h.
The excretion of this product is mainly through urine and feces, and 5% to 6% of the original drug is excreted in urine. The exact metabolic pathway of this product has not yet been determined. In vitro experiments have shown that it is mainly transformed by peptidase rather than CYP450 enzyme.
The pharmacokinetic properties of this product have nothing to do with liver and kidney function, body mass, and gender. However, the clearance rate of elderly men (>65 years old) is only 60% of that of young people, and that of elderly women is only 40% of that of young people.
Medicine interactions of Icatibatide Acetate
This product has not undergone formal drug interaction studies. In vitro experiments show that this product is not metabolized by cytochrome P450, and it is expected that this product will not have drug interactions with cytochrome P450 substrates, inhibitors and inducers.
How to buy Icatibant in the U. S.
USA Peptide supplier Remetide specializes in the production and sales of Icatibant Peptide, as well as professional drug peptide R&D. Feel free to contact us if you have any questions or inquiries about Peptides.