Unique peptides with potential anti-cancer capabilities have been found by Technion and University of Tokyo researchers. Unusual peptides have the potential to be effective anti-cancer treatments, according to a recent study published in Nature Communications. Attention has been drawn to peptides because of their possible application in the treatment of cancer. Peptides are little chains of amino acids joined together by peptide bonds.

Peptides only have a few dozens of amino acids, as opposed to proteins, which typically have hundreds. The cyclic peptides were found to, particularly link to ubiquitin protein chains, which are often utilized as a “death tag” for damaged proteins. The proteasome, sometimes known as the cell’s “trash can,” breaks down the proteins that have been labeled as damaged.
Proteins often include hundreds of amino acids, but peptides typically only contain a few dozen. Researchers have recently found cyclic peptides that can bind selectively to chains of ubiquitin proteins, which are frequently utilized as a “death tag” for damaged proteins. The proteasome, a component of the cell that removes garbage, subsequently breaks down these designated proteins.
Professor Ashraf Brik, Dr. Ganga B. Vamisetti, and Dr. Abbishek Saha from the Technion’s Schulich Faculty of Chemistry collaborated on the study with Professor Nabieh Ayoub from the Technion’s Faculty of Biology and Professor Hiroaki Suga from the University of Tokyo.
Three researchers, including Distinguished Professors Aharon Ciechanover and Avraham Hershko of the Technion’s Ruth and Bruce Rappaport Faculty of Medicine, were given the 2004 Nobel Prize in Chemistry for their discovery of the ubiquitin system.
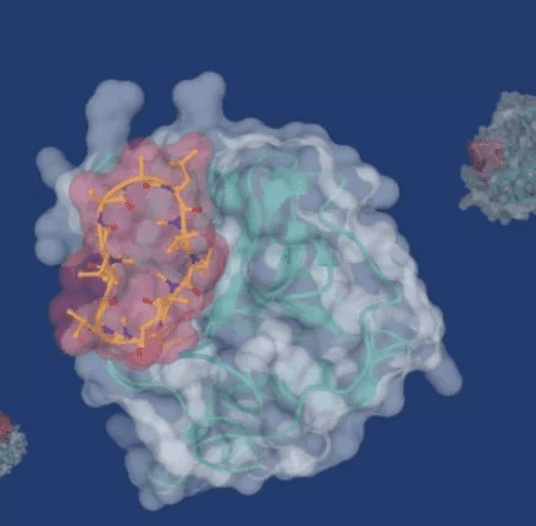
Over time, it became evident that the ubiquitin system’s function is somewhat influenced by the location of the chain’s links between individual ubiquitin molecules. For instance, joining the ubiquitin at position 48 (K48) in the chain causes proteins to be removed by the proteasome, whereas linking the ubiquitin at position 63 (K63) causes DNA damage to be repaired.
Technion researchers have created a fresh strategy for manipulating the ubiquitin processes recently. They chose to try a direct intervention in the ubiquitin chain rather than affecting the activity of enzymes that influence these pathways.
Based on this strategy, the researchers created cyclic peptides in a prior study that bind the K48-linked ubiquitin chains and stop them from causing the breakdown of the damaged proteins. Cells progressively begin to die as intended as a result of this disruption. They proposed and subsequently demonstrated in the same study that when such an event developed in a malignant tumor, it killed the cancer cells, potentially protecting the patient. This discovery, which was reported on in the 2019 issue of the journal Nature Chemistry, sparked the creation of a brand-new firm that is moving the discovery closer to clinical use.
The recent study identified cyclic peptides that are important in mending damaged DNA and binding the chains related to position 63 in ubiquitin. The scientists discovered that these peptides interfere with the aforementioned repair mechanism when bound to these ubiquitin chains. Cell death results from this and the accumulation of damaged DNA. Again, when this binding takes place in cancer cells, it kills those cells. According to the researchers, this therapeutic approach may be more successful than the currently available anti-cancer medications, to which patients gradually develop resistance.

