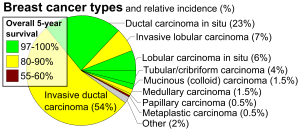
Latest FDA Regulations on Peptides Used as Bulk Substances for 503A or 503B Compounding Pharmacies
The FDA’s latest regulations on peptides are focused on ensuring the safety and efficacy of these drugs. In recent years, there has been a growing interest in peptides for the treatment of a variety of conditions, including cancer, diabetes, and obesity. However, peptides can also be complex and challenging to develop and manufacture. On September 29, 2023, FDA issued Safety Risks Associated with Certain Bulk Drug Substances Nominated for Use in Compounding, in which many popular peptides used in compounding pharmacies are considered not safe or